Suzanne Leigh, UC San Francisco

What if an over-the-counter allergy medicine could help halt and even reverse multiple sclerosis? And if it did, could patients return to their full capacity? Those were some of the questions first posed in 2013 after a landmark discovery by UC San Francisco neuroscientist Jonah Chan, Ph.D., and physician-scientist Ari Green, M.D.
The two had discovered in the lab that the medication clemastine — originally approved as an antihistamine — could repair myelin, the protective insulation around nerve fibers that is damaged in MS. This damage causes delays in nerve signals in the brain and central nervous system, leading to a cascade of MS symptoms that include muscle weakness and spasticity, vision loss, cognitive slowing, and incontinence. Despite years of research into the debilitating disease, even the most successful therapies had only slowed disease progression, not reversed it.
That early discovery in Chan’s lab, in which clemastine was shown to stimulate the differentiation of myelin-making stem cells, led to clinical trials to test the therapy in people. Starting with 50 patients in the five-month ReBUILD trial, Green showed that the allergy medication reduced the delayed nerve signaling in people with nerve damage from MS — a sign that what had been observed in the lab could result in an effective treatment in humans.
Surprisingly, these were patients with long-term damage from chronic MS, not just recent damage, said Green, medical director of the UCSF Multiple Sclerosis and Neuroinflammation Center, pointing to his team’s 2017 study in The Lancet. Achieving this outcome was equivalent, Green says, “to solving a crime months after the perpetrator had fled the crime scene.”
A search for proof
The question remained, though: did the medication work to repair myelin more broadly in the brain?
“We needed clearer biological evidence, and we knew we couldn’t turn to patient-reported outcomes. For some patients, feelings are understandably super-influenced by their optimism and hope,” said Green, who is also a neuro-ophthalmologist, chief of the Division of Neuroinflammation and Glial Biology in the UCSF Department of Neurology, and a member of the Weill Institute for Neurosciences.
So together with Chan and researchers Christian Cordano, M.D., Ph.D.; Eduardo Caverzasi, M.D.; and Nico Papinutto, Ph.D., they zeroed in on myelin-rich areas of the brain using MRIs from participants in their earlier trial.
The trial had split the patients into two groups: one set received clemastine in the first portion of the study and the other received clemastine in the second portion. The researchers found that the myelin content of the targeted areas they evaluated in the brain increased in group No. 1 during the first portion of the study and continued to increase in the second portion after clemastine was stopped. In group No. 2, myelin dropped in the first portion of the study and rebounded in the second portion. They had their proof.
Those MRI findings earned first author Cordano a coveted best poster award in October 2022 from the European Committee for Treatment and Research in Multiple Sclerosis, a Swiss-based nonprofit dedicated to the understanding and treatment of the disease.
Sea change in prognosis
The 2022 findings were accompanied and preceded by studies that reinforced that clemastine acted directly on myelin and proved the importance of myelin for keeping nerve fibers healthy. Meanwhile, labs around the world have confirmed Chan’s initial findings, and additional work from the ReBUILD trial, found that the treated patients had lower levels of a biomarker of nerve damage. Data also linked MS patients’ vision improvements to increases in myelin around the optic nerve.
New clinical trials in the UCSF Innovation Program for Remyelination and Repair are working to determine whether the MRI results can be independently confirmed, and whether other tools might be used to detect remyelination and track repair. The team is also studying clemastine’s effectiveness in acute painful vision loss and on active brain lesions in MS. And, together with their colleague Riley Bove, M.D., they are testing a different class of medications from Chan and Green’s initial screen as another possible treatment.
Collectively, this work may herald a sea change in prognosis for patients with MS. Before the advent in the 1990s of disease-modifying drugs that could delay MS progression, the greatest fear of newly diagnosed patients, who are typically in their 20s and 30s, was ending up in a wheelchair by their 40s.
Now, with revived hope that clemastine — or a similar, yet-to-be developed drug — may restore some function, Green believes it’s time for neurologists to shift attention away from canes and wheelchairs.
“Long-term patients cannot walk into a darkened theater because they cannot see well enough to find a seat. They cannot look through a bright window. Their memory, attention and judgment are not quite the same,” he said. “For years we’ve rightfully focused on keeping patients out of wheelchairs. We’re hoping that if we can restore some function, we can focus on these smaller, less obvious disabilities.”
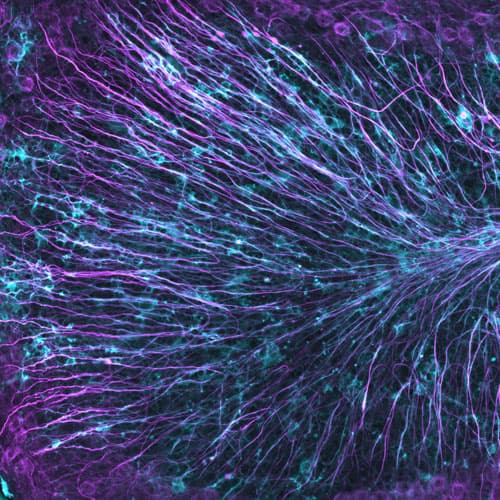
Multiple sclerosis often results in significant loss of myelin, the protective insulation around nerve fibers, causing delays in nerve signals in the brain and central nervous system.
Researchers needed proof that clamestine, an over-the-counter allergy medication, could increase myelin content in the brain.

