Wayne Lewis, UCLA

Peanut allergies affect 1 in 50 children, and the most severe cases lead to a potentially deadly immune reaction called anaphylactic shock.
Currently, there is only one approved treatment that reduces the severity of the allergic reaction, and it takes months to kick in. A group of UCLA immunologists is aiming to change that.
Taking inspiration from COVID-19 vaccines as well as their own research on the disease, they created a first-of-its-kind nanoparticle — so small it’s measured in billionths of a meter — that delivers mRNA to specific cells in the liver. Those cells, in turn, teach the body’s natural defenses to tolerate peanut proteins.
In testing in mice, the nanoparticle not only reversed peanut allergies, but also prevented them from developing. The study was published in the journal ACS Nano.
“As far as we can find, mRNA has never been used for an allergic disease,” said Dr. André Nel, the paper’s co-corresponding author, a UCLA distinguished professor of medicine and director of research at the California NanoSystems Institute at UCLA. “We’ve shown that our platform can work to calm peanut allergies, and we believe it may be able to do the same for other allergens, in food and drugs, as well as autoimmune conditions.”
The researchers focused on the liver for two reasons: First, the organ is trained not to respond to every challenge because it is regularly bombarded with foreign substances, including allergens. Second, the organ is home to cells called antigen-presenting cells, which collect foreign proteins and train the immune system to tolerate them rather than attacking when they’re detected.
The study builds on two previous advances from Nel and his colleagues. In 2021, they found that a nanoparticle delivering a carefully selected protein fragment, called an epitope, to the liver reduced symptoms of dangerous egg allergy in mice. The following year, they identified one epitope that alleviated peanut allergies in mice when delivered to the liver via a nanoparticle. Because these epitopes leave out the part of the peanut or egg protein that triggers allergies, they’re expected to be safer as part of a treatment.
“If you’re lucky enough to choose the correct epitope, there’s an immune mechanism that puts a damper on reactions to all of the other fragments,” said Nel, who also directs the University of California’s Center for Environmental Implications of Nanotechnology, or CEIN. “That way, you could take care of a whole ensemble of epitopes that play a role in disease.”
The scientists improved on the design of their previous nanoparticle by adding a sugar molecule on its surface that specifically binds to antigen-presenting cells. Using mRNA was another step forward.
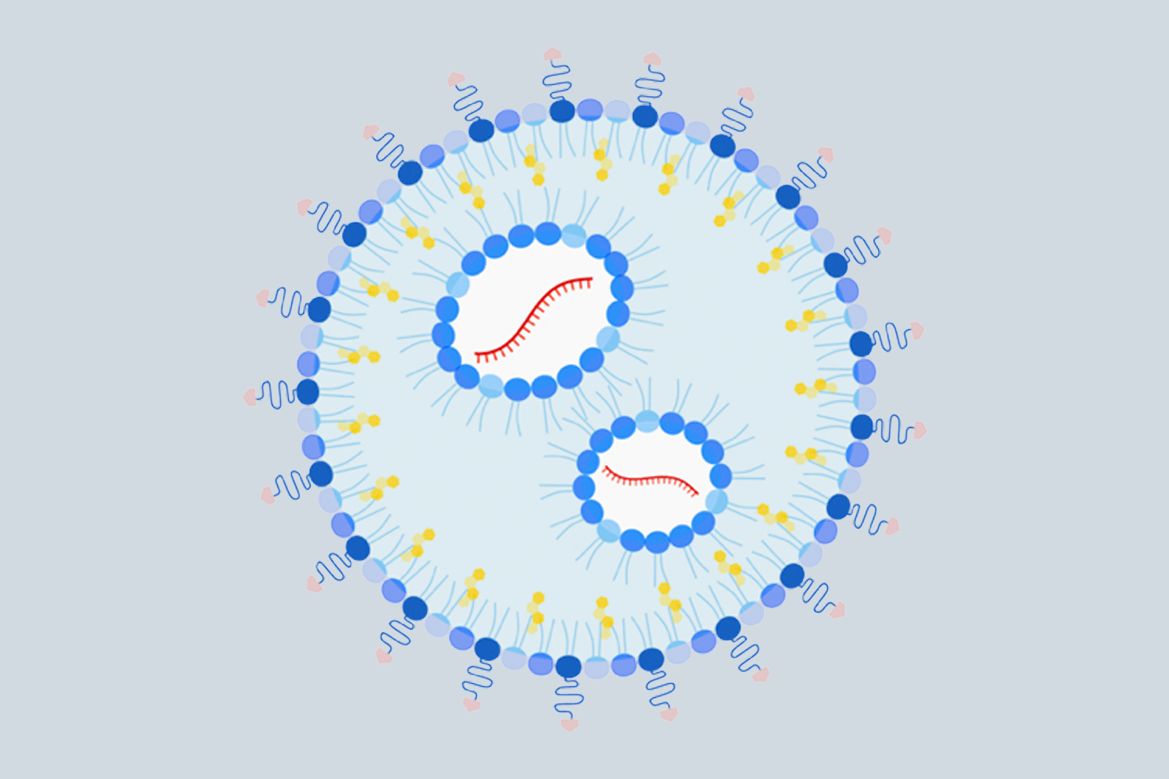
In the upgraded nanoparticle, the investigators designed part of the mRNA payload to encode the selected epitope or epitopes — in this case, the peanut protein fragment identified in a previous study — the same way that mRNA vaccines for SARS-CoV-2 encode the entire spike protein of the virus. Using mRNA makes it easier to load the nanoparticle and eliminates the complications that come with including more than one epitope, an advantage that may expand the scope of application. For instance, multiple epitopes might be needed to address certain other allergies, or multiple allergies.
To evaluate whether their upgraded nanoparticle would prevent peanut allergies, the researchers gave it to six mice in two doses, a week apart. Another group of six mice got a nanoparticle with the same mRNA payload, but with no targeting sugar on its surface; six other mice got the upgraded nanoparticle but with mRNA inside that didn’t code for any protein or epitope; and a third group of six got no nanoparticle at all. Starting one week after the second dose, they fed the mice a crude peanut protein extract to sensitize them to the peanut allergens. Another week later, they exposed the mice to peanut protein to trigger anaphylactic shock.
Mice that were pretreated with the upgraded nanoparticle showed milder symptoms compared to those who received a nanoparticle with no targeting sugar, while more-serious symptoms appeared in the control group receiving no treatment and the group getting a targeted nanoparticle with noncoding mRNA.
The scientists repeated the experiment, changing the order of operations — so that mice were sensitized to peanut protein before receiving the nanoparticle. Again, the upgraded nanoparticle outperformed a similar one that lacked the targeting sugar, and both produced far milder symptoms than the researchers observed in mice given no treatment or a nanoparticle containing noncoding mRNA.
In both versions of the experiment, the scientists measured the levels of specific immune cells as well as certain antibodies, enzymes and cytokines, which confirmed that the upgraded nanoparticle had increased the animals’ tolerance for peanut protein.
Nel estimates that, with success in further lab studies, the nanoparticle could be in clinical trials within three years. (His lab will soon begin the regulatory process that’s required to test the approach for peanut allergies in clinical trials.) He added that substituting an mRNA payload coding for different epitopes opens up the potential to adapt the nanoparticle for other allergies and autoimmune disorders.
The team is exploring whether the nanoparticle could be used to treat type 1 diabetes, a disease in which the immune system attacks cells in the pancreas that enable the body to get energy from food. Important epitopes from the proteins that trigger the immune attack in diabetes have already been identified by other researchers.
The study’s co-first authors are Xiao Xu, a UCLA postdoctoral scholar, and Dr. Xiang Wang, a UCLA senior researcher. Dr. Tian Xia, a UCLA associate adjunct professor of nanomedicine, is co-corresponding author. Other authors are senior researcher Yu-Pei Liao and postdoctoral scholar Lijia Luo, both of UCLA.
The study was funded by the National Institutes of Health, the Marlin Miller Jr. Family Foundation and the Noble Family Innovation Fund at CNSI.

