Susanne Clara Bard, UC San Diego
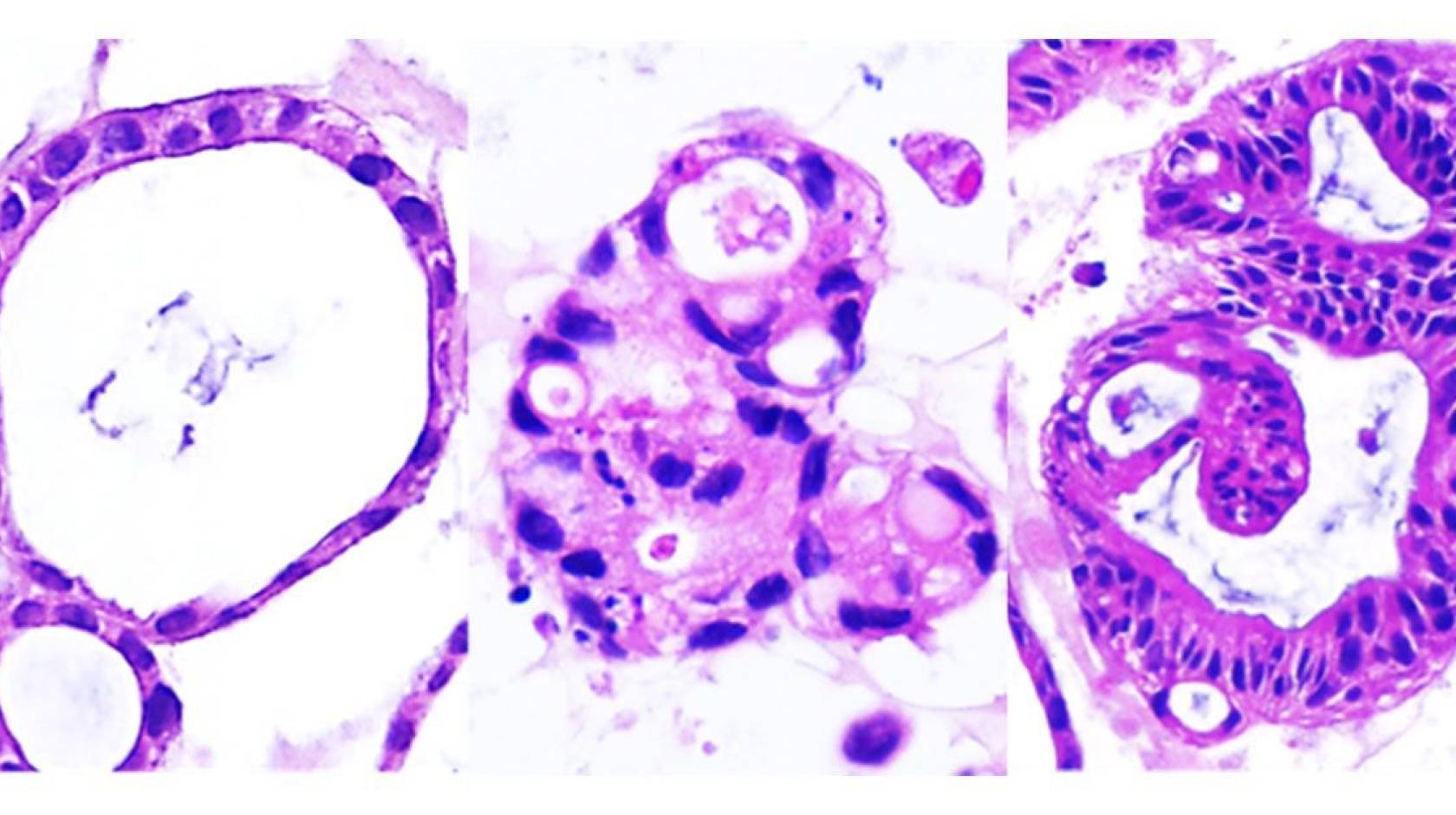
Crohn’s disease — an autoimmune disorder — is characterized by chronic inflammation of the digestive tract, resulting in a slew of debilitating gastrointestinal symptoms that vary from patient to patient. Complications of the disease can destroy the gut lining, requiring repeated surgeries. The poorly understood condition, which currently has no cure and few treatment options, often strikes young people, causing significant ill-health throughout their lifetime.
One barrier to making progress in developing treatments has been the lack of preclinical animal models that accurately replicate this complex disease. Another is the extreme heterogeneity among patients in the clinic, making it difficult for clinicians to tailor therapies.
Previous studies of Crohn’s disease have derived organoids from pluripotent stem cells reprogrammed to become gut cells. Now, by studying organoids derived from adult stem cells in the gut tissue of Crohn’s disease patients — which more accurately replicated the traits of the disease — University of California San Diego researchers have discovered that the condition consists of two distinct molecular subtypes.
The findings, published in Cell Reports Medicine on September 26, 2024, could lay the foundation for improved diagnostics and the development of personalized treatment options based on which subtype a patient’s disease falls into.
The researchers sampled gut tissue from 53 Crohn’s disease patients during routine colonoscopies at the Inflammatory Bowel Disease Center at UC San Diego Health. The patients came from diverse backgrounds and presented with a variety of clinical symptoms. Scientists at the UC San Diego HUMANOIDTM Center of Research Excellence at UC San Diego School of Medicine, led by its director and the first author of the study, Courtney Tindle, created a “biobank” of patient-derived organoid cultures.
Organoids are tiny, lab-grown replicas of organs or tissues that closely mimic the behavior of their real-life counterparts. They are especially useful in medical research when animal models do not adequately represent the disease.
Previous studies of Crohn’s disease have derived organoids from pluripotent stem cells reprogrammed to become gut cells. However, developing the organoids from adult stem cells in gut tissue more accurately replicates the traits of the disease, according to senior author Pradipta Ghosh, M.D., professor of cellular and molecular medicine and executive director of the HUMANOIDTM Center.
“The pluripotent stem cells — whether derived from blood or skin cells, carry the genetic traits of the patient, but have no way to know the inflamed environment inside the patient’s gut,” said Ghosh. In contrast, the adult stem cells retain an epigenetic memory of the gut environment imprinted on the genetic background — including a history of bacterial colonization, inflammation, and altered oxygen and pH. “We show here that adult stem cell-derived organoids accurately mimic the inflamed gut, but the pluripotent cells fail, which reminds me of what Maya Angelou once said: ‘I have great respect for the past. If you don't know where you've come from, you don't know where you're going,’” she added.
Upon analysis, the researchers were surprised to discover that no matter how many clinically diverse patients they recruited, the organoids consistently fell into one of just two discrete molecular subtypes — each exhibiting unique patterns of genetic mutation, gene expression, and cellular phenotypes.
The two subtypes are:
-
Immune-deficient infectious-Crohn’s disease (IDICD), characterized by difficulty clearing pathogens, and an insufficient cytokine response by the immune system. These patients tend to form fistulas with pus discharge.
-
Stress and senescence-induced fibrostenotic-Crohn’s disease (S2FCD), characterized by cellular aging and stress. These patients tend to develop fibrosis — or scarring — of gut epithelial tissue, which may form strictures.
Ghosh believes the discovery of these two distinct molecular subtypes will lead to a shift in the traditional understanding of Crohn’s disease. Instead of classifying patients based on a large array of clinical symptoms, categorizing them by one of the two molecular subtypes could open the door to more personalized and effective management strategies.

Pradipta Ghosh, M.D., professor of cellular and molecular medicine and executive director of the HUMANOIDTM Center.
“Currently, because of a lack of understanding of these fundamentally different subtypes, they are all being given the same treatment — a cookie-cutter therapy,” said Ghosh. “This combination of anti-inflammatory drugs helps a fraction of the patients, but only temporarily.”
The study revealed that patients with immune-deficient infectious-Crohn’s disease might be better served by therapies that clear their bacterial infections instead.
In contrast, for those with the stress and senescence-induced fibrostenotic-Crohn’s disease subtype, Ghosh says drugs that target or reverse cellular aging — cell-based and stem cell-based therapies that rejuvenate the gut epithelium — might be more effective at treating their disease.
Testing different drugs on patient-derived organoids will help identify which drugs are most effective for each molecular subtype.
She adds that work is underway to genotype the two subtypes in order to develop a simple test that can quickly identify which subtype a patient falls into.
“It’s my hope that when the genetics are completed, Crohn's disease will be viewed as two molecular subtypes that should be treated in two completely different ways.”
Ghosh says future treatments for Crohn’s disease — which affects more than 500,000 Americans — could one day include personalized cell therapies, including gene editing and RNA-based treatments.
“Traditionally, we have been treating this disease with anti-inflammatory drugs, an approach that can be likened to putting out fires. With this study — the first of many based on the ongoing work and the efforts that are going to translate this to the clinic — we hope to target the arsonist who is responsible for the fire in the first place.”
Ghosh says the logistics and infrastructure of HUMANOIDTM and the Institute for Network Medicine uniquely enables bold disease modeling efforts within a collaborative framework that extends from the laboratory bench to the clinic and the cloud. The team partnered with gastroenterologists William Sandborn, M.D. and Brigid S. Boland, M.D. at the Inflammatory Bowel Disease Center at UC San Diego Health, who advised them on disease modeling, and with data scientists who determined how closely the model matched the disease.
Co-authors on the study include: Ayden G. Fonseca, Sahar Taheri, Gajanan D. Katkar, Jasper Lee, Priti Maity, Ibrahim M. Sayed, Stella-Rita Ibeawuchi, Eleadah Vidales, Rama F. Pranadinata, Mackenzie Fuller, Dominik L. Stec, Mahitha Shree Anandachar, Kevin Perry, Helen N. Le, Jason Ear, Debashis Sahoo, and Soumita Das, all at UC San Diego.
The study was funded, in part, by the Leona M. and Harry B. Helmsley Charitable Trust, the National Institutes of Health (Grants AI141630, DK107585, R56 AG069689, DiaComp Pilot and Feasibility award, R01-GM138385), and Padres Pedal the Cause/C3 Collaborative Translational Cancer Research Award (San Diego NCI Cancer Centers Council [C3] #PTC2017).
Financial interests disclosure: Soumita Das and Pradipta Ghosh hold a patent on the methodology described in the paper. The authors declare no other competing interests.

