Sonia Fernandez, UC Santa Barbara
About a decade ago, researchers in UC Santa Barbara chemistry professor Guillermo Bazan’s lab began to observe a recurring challenge in their research: Some of the compounds they were developing to harness energy from bacteria were instead killing the microbes. Not good if the objective of the project was to harness the metabolism of living bacteria to produce electricity.
“We needed the bacteria to be alive,” said Alex Moreland, a Cystic Fibrosis Foundation Postdoctoral Fellow who joined the Bazan research group as a graduate student in 2014, and currently works at UCSB’s Center for Polymers and Organic Solids. “While we were developing new molecules for that application, we found that some of them didn’t work because they were killing the bacteria.”
However, instead of brushing it off as a rather annoying laboratory curiosity, in subsequent research the team leaned into the apparent antimicrobial properties of these compounds, called conjugated oligoelectrolytes (COE). Fast-forward to today, and they now have the basis for a new class of antibiotics, one that not only shows promise against a broad array of bacterial infections but can also evade the dreaded resistance that has been rendering our current generation of first-line antibiotics ineffective.
“We realized that the molecular frameworks that we had been working on for some time could, if properly designed, yield a new class of antibiotics; something that is seldom found and that has profound implications for modern medicine,” Bazan said.
The Bazan Group’s proof-of-concept studies for a wide range of bacterial infections appear across multiple papers published in Science Translational Medicine, the Journal of Medicinal Chemistry, and Chemical Communications.
A global problem
In what has been called an overlooked pandemic, antimicrobial resistance (AMR) is a global problem that affects all walks of life. In 2019, an estimated 1.3 million deaths around the globe could be attributed to AMR.
“This figure assumes that if the resistant bacteria was replaced with a non-resistant bacteria of the same type, the patients would have survived,” Moreland said. “These are excess deaths specifically related to resistance to antibiotics that were effective in years past.” In many cases, he added, the mortality rate for infections with certain resistant bacteria is more than three times higher than that for non-resistant strains.
Antibiotic resistance develops when bacteria are exposed to an antibiotic and evolve ways to defeat or bypass the antibiotic. Strategies include using the cell membrane as a barrier, destroying the offending molecule or eliminating it from the cell, or altering the drug’s target to render the antibiotic ineffective. These resistance mechanisms can be passed on to progeny bacteria or shared with other bacteria in the environment.
“There were 4.95 million deaths associated with antibiotic resistance in 2019, including the 1.3 million deaths that could be directly attributed to AMR, while around 10 million people die every year from cancer,” Bazan commented. “However, last time we checked, there were 27 clinical trials for new antibiotics and 1,300 for anticancer treatments. It is worth taking a moment to reflect on these numbers.”
Broadly effective, yet highly selective
COEs appear to hit multiple targets by “remodeling” bacterial membranes, the international team of researchers demonstrate in Science Translational Medicine. Led by Kaixi Zhang, at the time a National University of Singapore (NUS) postdoctoral researcher in the Bazan Lab, the team deployed their compounds against a particularly difficult-to-treat microbe, Mycobacterium abscessus (Mab), infections of which are prevalent in patients with underlying lung diseases, such as cystic fibrosis. Not only does Mab have “an unusually thick and impermeable cell envelope” that repels antibiotics, it also has the ability to hide inside phagocytes, immune cells whose job it is to engulf and kill microorganisms.
In the case of Mab, these immune cells do not efficiently kill the bacteria and may unintentionally harbor them against antibiotics. Current treatments often fail despite long bouts with three to four antibiotic combinations for 12 to 18 months — more than half of the patients are not cured, yet more than 70% of the patients suffer from notable adverse side effects. The COE in this study proved more effective than antibiotic controls amikacin and imipenem at eradicating Mab in both in-vitro and in-vivo experiments.

Guillermo Bazan.
The researchers attribute this effectiveness to the compound’s targeting of the physical and functional integrity of the bacteria’s cell wall.
“If you destroy the membrane, the cell will rupture and of course that’s going to kill the bacteria, but that tends not to be a selective mechanism,” Zhang said. “However, there are a lot of essential functions that happen in the membrane that can be interrupted by more subtle membrane targeting. Our hypothesis is that our compounds, by inducing membrane remodeling, inhibit multiple essential functions simultaneously.” This onslaught of disruption has a multiplicative effect on the bacteria, she added, making it 10 to 1000 times more difficult for them to develop resistance compared to exposure to conventional antibiotics.
The unique mechanism of COEs also figures heavily in another facet of antibiotic resistance or tolerance: the production of a biofilm, a state in which a community of microbes band together and produce a polymeric substance, creating a shield of sorts.
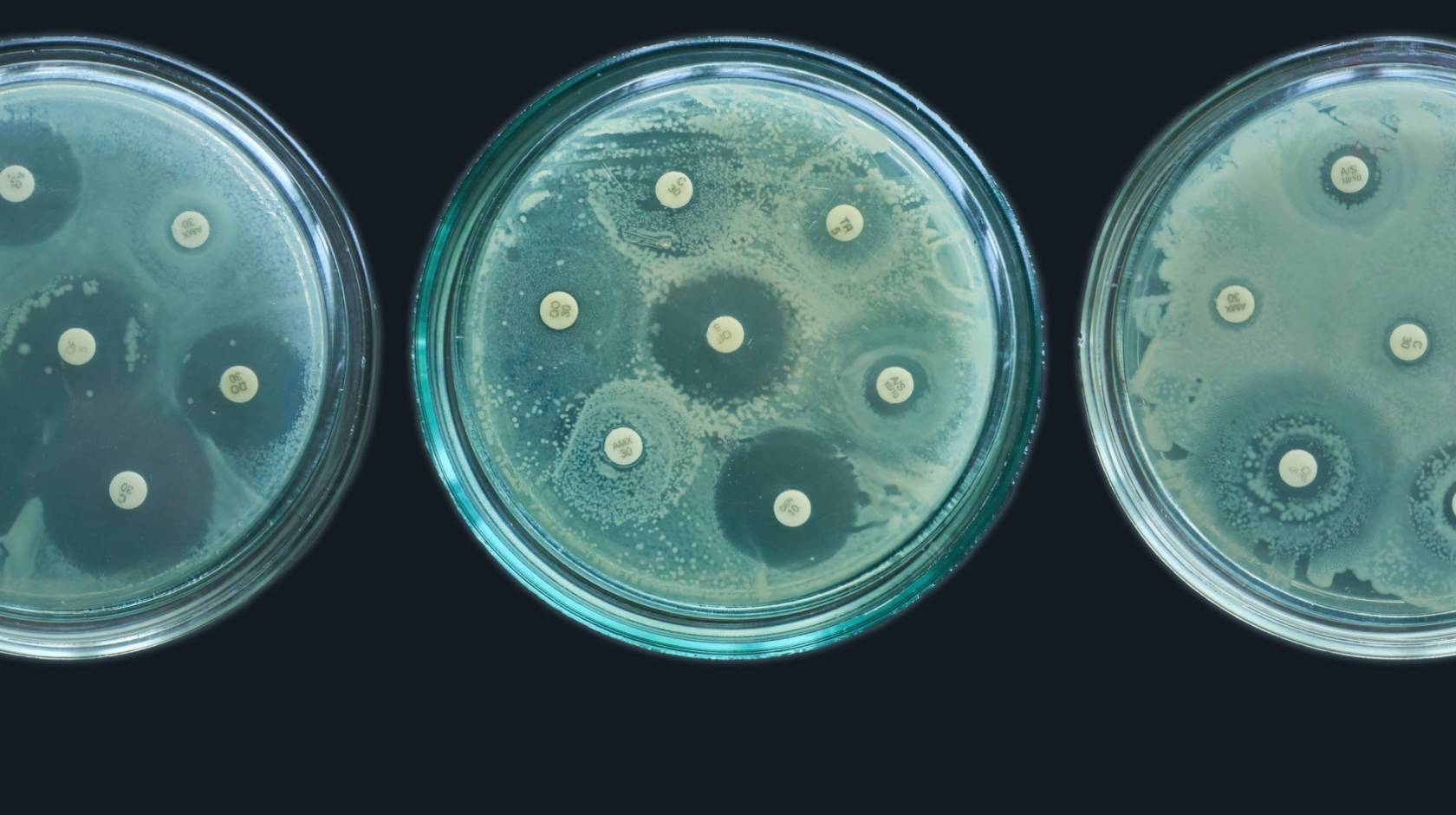
In the Journal of Medicinal Chemistry, led by UCSB/NUS postdoctoral researcher Jakkarin Limwongyut, the team demonstrated another COE compound’s efficacy against Pseudomonas aeruginosa, a biofilm-forming drug-resistant bacteria that is considered an urgent threat by World Health Organization and the Centers for Disease Control and Prevention, and is among the pathogens more traditionally associated with AMR. It causes a variety of diseases from ear infections to life-threatening pneumonia, and is especially prevalent in hospital settings.
“Some antibiotics can’t penetrate a biofilm, but also when bacteria form biofilms, their metabolism changes because they have less access to nutrients,” said Limwongyut, explaining that the slower metabolism can render the effects of an antibiotic more tolerable to the pathogens and therefore less effective. “Recalcitrant and recurring infections, be that UTIs, pneumonia, endocarditis, or diabetic foot ulcer infections, are often associated with biofilms,” he said.
The team proved that their COE compound was capable of killing bacteria in established biofilms while also inhibiting the formation of biofilms. It’s a rare one-two punch in the world of antibiotics.
“There are a couple of antibiotics that do have anti-biofilm activity, but they either aren’t used systemically or they are used systemically but really shouldn’t be,” Moreland said, alluding to the high toxicity of some of these antibiotics. For example, polymyxins in a topical form are effective against biofilms, but are toxic to kidneys at the doses used systemically (intravenous injection). Polymyxins accumulate in patients’ kidneys, causing damage to the cells and tissue, and in severe cases, leading to kidney transplants.
In contrast, the Bazan Lab has developed COEs to be highly selective for bacteria. In Chemical Communications, Moreland and team investigated how structural features of these molecules could drive their affinity for bacterial membranes and their antibiotic activity without “detergent-like” effects. In detergents, the antibacterial action relies on the indiscriminate destruction of cell membranes.
“Your skin cells are pretty good at tolerating soaps and detergents but other cells in your body, and especially red blood cells, are very sensitive,” he said, which is why these compounds are used only externally or to decontaminate surfaces and not as therapeutic agents. For COEs, they found, membrane permeability and antibiotic action are not inherently linked,
suggesting a novel mechanism behind the COEs’ activity, and critically, a mechanism that can be highly selective for bacterial membranes over mammalian ones. In fact, the molecules in the Mab experiment were able to reach inside of the phagocytes to kill the bacteria without damaging the mammalian cells.
“We don’t yet know the exact mechanisms, but we can definitively show that COEs kill bacteria and don’t kill mammalian cells,” Moreland said. He added, “this was not necessarily the case with the original molecules that we discovered early on, but with a lot of chemistry, and the help of tools such as machine learning, we were able to determine which molecular structures appear to strike the balance between efficacy against bacteria and safety for mammals.” In various infection models, mice also appeared to tolerate COE treatments fairly easily.
Looking ahead
It’s still early days for the Bazan research group, now mostly based in Singapore, as they continue to investigate mechanisms of action, search for additional novel properties and design and refine their molecules. Ideally, COE antibiotics would someday serve as safe and effective treatments, effective in cases of even the most resistant bacterial infections. Still, the road to clinical trials is a long one, albeit with interest and support from a variety of institutes and research collaborations around the globe, from the Singapore Centre for Environmental Life Sciences to the Cystic Fibrosis Foundation and Walter Reed Army Institute of Research in the U.S.
“So far, so good. COEs have worked well in the experiments that we’ve done to-date,” said Moreland, adding that the molecules in the studies need further refinement before advancing into clinical trials. “There’s obviously more development required but we’re up to it.”

Alex Moreland with a plate of Pseudomonas aeruginosa at the UCSB California Nanosystems Institute (CNSI) Biological Nanostructures Laboratory (BNL).