Iqbal Pittalwala, UC Riverside

Zika virus (ZIKV), which causes Zika virus disease, is spread to people primarily through the bite of an infected Aedes aegypti or Aedes albopictus mosquito. An infected pregnant woman can pass ZIKV to her fetus during pregnancy or around the time of birth. Sex is yet another way for infected persons to transmit ZIKV to others.
Credit: Song Lab/UC Riverside
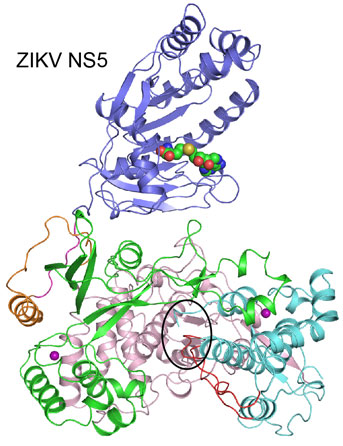
The genomic replication of the virus is made possible by its “NS5” protein. This function of ZIKV NS5 is unique to the virus, making it an ideal target for anti-viral drug development. Currently, there is no vaccine or medicine to fight ZIKV infection.
In a research paper just published in Nature Communications, University of California, Riverside scientists report that they have determined the crystal structure of the entire ZIKV NS5 protein and demonstrated that NS5 is functional when purified in vitro. Knowing the structure of ZIKV NS5 helps the researchers understand how ZIKV replicates itself.
Furthermore, the researchers’ structural analysis of ZIKV NS5 reveals a potential binding site in the protein for an inhibitor, thereby providing a strong basis for developing potential inhibitors against ZIKV NS5 to suppress ZIKV infection. The identification of the inhibitor-binding site of NS5 can now enable scientists to design potential potent drugs to fight ZIKV.
“We started this work realizing that the full structure of ZIKV NS5 was missing,” said Jikui Song, an assistant professor of biochemistry, who co-led the research with Rong Hai, an assistant professor of plant pathology and microbiology. “The main challenge for us occurred during the protein’s purification process when ZIKV NS5 got degraded — chopped up — by bacterial enzymes.”
Opens the door to drug development

Credit: Iqbal Pittalwala/UC Riverside
Song, Hai and their colleagues overcame this challenge by developing an efficient protocol for protein purification, which in essence minimizes the purification time for NS5.
“Our work provides a framework for future studies of ZIKV NS5 and opportunities for drug development against ZIKV based on its structural similarity to the NS5 protein of other flaviviruses, such as the dengue virus,” Hai said. “No doubt, ZIKV therapeutics can benefit from the wealth of knowledge that has already been generated in the dengue virus field.”
Next, the researchers plan to investigate the antiviral potential on ZIKV NS5 of a chemical compound that has been shown to work effectively in inhibiting the NS5 protein in the dengue virus.
Song and Hai were joined in the research by graduate students Boxiao Wang (first author), Xiao-Feng Tan, Stephanie Thurmond, Zhi-Min Zhang, and Asher Lin.
The research was supported by grants to Song from the March of Dimes Foundation, the Sidney Kimmel Foundation for Cancer Research and the National Institutes of Health.

