Nicole Feldman, UC Irvine
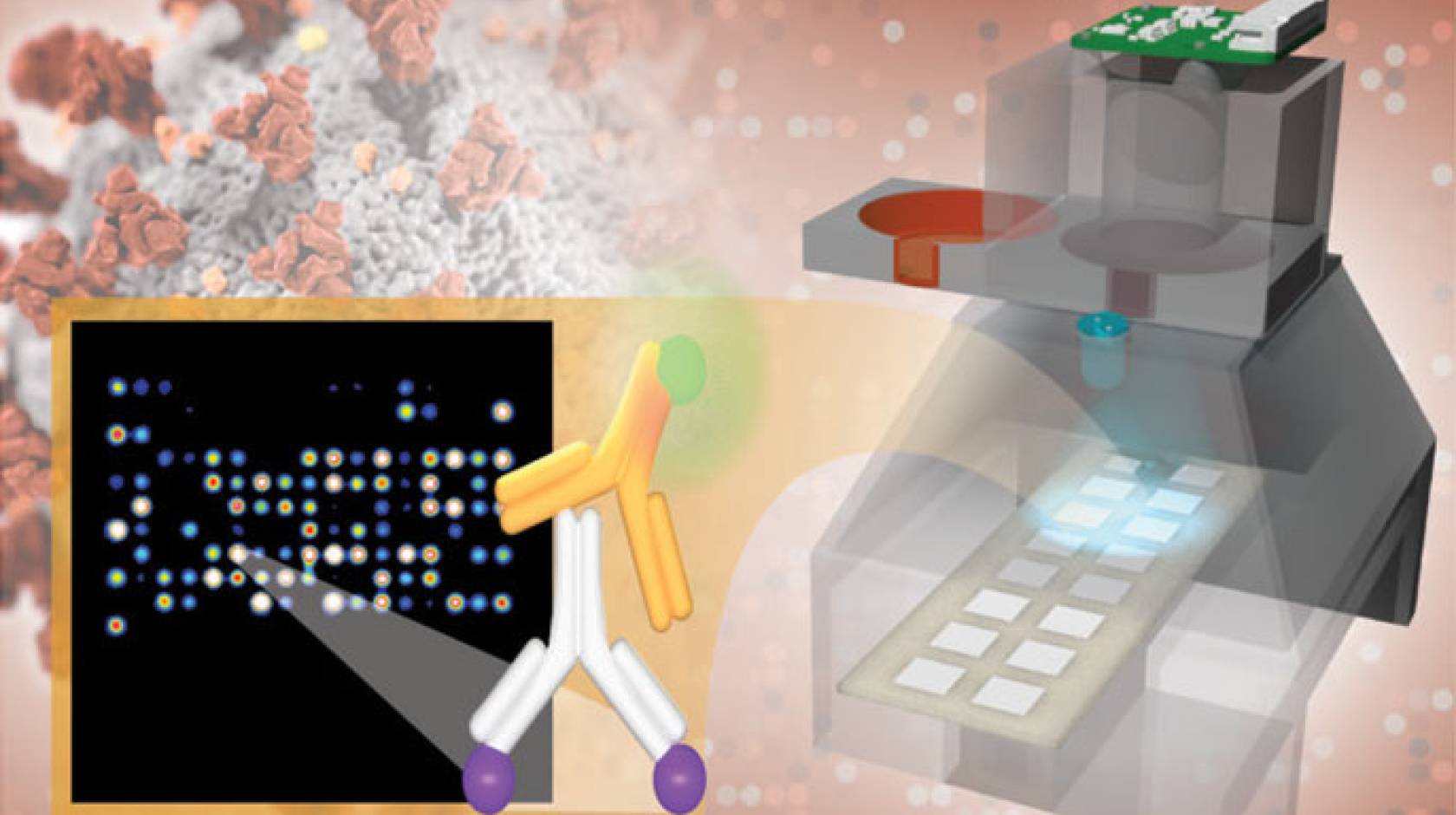
A robust, low-cost imaging platform utilizing lab-on-a-chip technology created by University of California, Irvine scientists may be available for rapid coronavirus diagnostic and antibody testing throughout the nation by the end of the year.
The UC Irvine system can go a long way toward the deployment of a vaccine for COVID-19 and toward reopening the economy, as both require widespread testing for the virus and its antibodies. So far, antibody testing in the U.S. has been too inaccurate or expensive to reach the necessary numbers.
But UC Irvine investigators Weian Zhao, Per Niklas Hedde, Enrico Gratton and Philip Felgner believe that their new technology can help accelerate the testing process quickly and affordably. Their discovery appears in the journal Lab on a Chip, which is published by the Royal Society of Chemistry.
“We need to test millions of people a day, and we’re very far from that,” said Hedde, a project scientist in pharmaceutical sciences and the study’s lead author. “This accurate testing platform enables public health officers to implement individualized mitigation strategies that are needed to safely reopen the country and economy.”
How it works
Using blood from a finger prick, the UC Irvine test probes hundreds of antibody responses to 14 respiratory viruses, including SARS-CoV-2, in a mere two to four hours. Identifying responses to viral infections with symptoms similar to those of COVID-19 will keep hospitals clear of patients with standard colds and flus.
The results are printed on a low-cost imaging platform. The TinyArray imager combines a 3D-printed prototype with an off-the-shelf LED and a small 5-megapixel camera to find markers for many antibodies simultaneously. This ensures accuracy equal to that of expensive imaging systems but makes the platform portable enough to deploy anywhere — at a cost of only $200.
The same device can also process the results of commonly used nose swab tests for SARS-CoV-2 so that patients can be tested for COVID-19 and its antibodies on a single platform.
Currently, most antibody tests only check for one or two antigens, the foreign substances that cause the body to produce antibodies.
“A month or two ago, testing was kind of regarded as the Wild West,” said Zhao, a professor of pharmaceutical sciences, adding that most SARS-CoV-2 antibody tests are “just not accurate.”
Systems that test for the full range of antibodies necessary for reliable results require imaging machines that cost $10,000 to $100,000 and are too bulky for widespread use. Areas without the resources to acquire one of these machines have to send their samples to external labs for testing, meaning that results take days instead of hours.
Big impact
Large-scale testing will determine what percentage of the population had COVID-19 but never showed symptoms, which will have a big impact on public health and reopening decisions.
“What if it turns out that a larger percentage of the people in a community have already contracted the virus?” Zhao said. “This means you are closer to accomplishing herd immunity.”
And understanding what antibodies are produced and how long they last will be key in developing an effective vaccine and administering the right dosage. This may be critical for years to come if the virus mutates, requiring updates much like yearly flu vaccinations.
The UC Irvine team has already completed 5,000 tests in Orange County, and the final goal is to test 20,000 samples per unit a day. The researchers are partnering with UC Irvine startups Velox Biosystems Inc. and Nanommune Inc. to scale up production. They expect that the TinyArray imager will be ready to deploy across the U.S. by the end of 2020 and are working with scientists in Uruguay, Russia and Thailand to develop similar systems for their nations.
“This would be great for a low-income country,” Hedde said. “Because the device’s materials are cheap and easy to obtain, the platform is easy to manufacture and use in low-resource areas, making testing accessible on a world scale.”
Aarti Jain, Rie Nakajima, Rafael Ramiro de Assis, Trevor Pearce, Algis Jasinskas and Saahir Khan of UC Irvine along with Timothy Abram and Melody Toosky of Velox Biosystems participated in the study, which was supported by the National Institutes of Health (grants P41 GM103540 and R01 AI117061) and a UCI CRAFT-COVID grant.
