Scott LaFee and Nicole Mlynaryk, Pat Harriman, UC San Diego, UC Irvine

With a five-year, $126 million grant from the National Institutes of Health (NIH), a multi-institution team of researchers at University of California San Diego School of Medicine, UC Irvine, Salk Institute for Biological Studies and Washington University in St. Louis has launched a new Center for Multiomic Human Brain Cell Atlas.
The center is the latest addition to the NIH’s Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative, which seeks to describe the human brain’s cells in unprecedented molecular detail, classifying them into more precise subtypes, pinpointing their locations in the brain and tracking how cellular features change over a lifetime.
“The goal is to better understand how neurotypical human brains work and age,” said Bing Ren, Ph.D., UC San Diego professor of cellular and molecular medicine and one of the effort’s principal investigators.
“The project will establish a baseline against which scientists will be able to compare brains with neurological or psychiatric conditions, such as Alzheimer’s disease, autism, depression and traumatic brain injury which, in turn, can lead to new insights, treatments and therapeutics.”
For example, said the center’s leader Joseph Ecker, Ph.D., director of the Genomic Analysis Laboratory at Salk and an adjunct professor of cell and developmental biology at UC San Diego School of Medicine, “we could say ‘that’s the region of the genome, in that specific subset of neurons, in that part of the brain, where a molecular event goes awry to cause that disease.’
“Ultimately this information might help us design gene therapies that target only the cell populations where the treatment is needed — delivering the right genes to the right place at the right time.”
In addition to Ren and Ecker, the Center for Multiomic Human Brain Cell Atlas includes Margarita Behrens, Ph.D., a research professor at Salk; Xiangmin Xu, Ph.D., professor of anatomy and neurobiology at UC Irvine; and Ting Wang, Ph.D., professor of medicine at Washington University School of Medicine in St. Louis.
From mice to men
The Center for Multiomic Human Brain Cell Atlas is part of the NIH’s BRAIN Initiative Cell Atlas Network (BICAN), expanding a five-year effort that began with mapping the mouse brain. The BRAIN Initiative Cell Census Network, which also involved Ren and Ecker, published its mouse atlas findings in a special issue of Nature in October 2021.
“Similar to the way we learned about space travel from short trips to the moon, the mouse brain mapping project taught us a lot about how to approach a much bigger brain, and the types of genomic information we would need to be able to truly map the human brain,” said Behrens at the Salk. “This project is an example of how fruitful teamwork can be in science — these types of projects cannot be accomplished in a single lab.”
Mapping the human brain
In the new project, researchers will examine 1,500 brain samples (50 regions each in 30 human brains ranging in age). The center will focus not only on gene expression patterns of each cell, but also DNA methylation, chromatin organization and histone modifications in the nucleus — molecular events that influence whether genes are turned “on” or “off” in a given cell type or at a particular time.
UC Irvine team members will primarily manage brain sample acquisition. As a full member of BICAN, UC Irvine will receive $10 million to collect, process and characterize a broad range of adult brain specimens. An interdisciplinary UC Irvine team, led by Xiangmin Xu, Ph.D., Chancellor’s Fellow of anatomy & neurobiology and director of the Center for Neural Circuit Mapping, will collaborate with scientists from the other three institutions.

“We are pleased to participate in this very large-scale mapping project to identify individual brain cells’ molecular features, location and how they change over time,” said Xu, UCI principal investigator. “This precise description will advance our understanding of how individual cells and complex neural networks interact in both space and time, leading to new ways to treat and prevent disorders such as Alzheimer’s, autism, depression and traumatic brain injury. It will also create a new foundational network for analyzing health and disease structure and function.”
This is the latest in a series of large BRAIN Initiative projects coordinated by Xu and will leverage the Center for Neural Circuit Mapping’s strong infrastructure and extensive expertise. The team will partner with the Department of Pathology & Laboratory Medicine’s Experimental Tissue Resource and the Alzheimer’s Disease Research Center at UC Irvine to obtain racially diverse samples from donors between 18 and 60 years old. These will be used for single-cell multiomics studies to gain a more comprehensive understanding of molecular changes contributing to normal development, cellular response and disease in human aging. (Read more about the UC Irvine team here.)
Salk scientists will isolate each nucleus from each individual cell in each region and record molecular details, such as chromatin architecture and DNA methylation. Chromatin architecture enables regulatory elements to communicate with genes located at a distance. DNA methylation is the process by which DNA is altered by chemical tags, changing gene expression.
Ren’s team includes Quan Zhu, Ph.D., and Nathan Zemke, Ph.D., both associate directors at the Center for Epigenomics in the UC San Diego School of Medicine, a collaborative research center that specializes in development and application of leading epigenomics technologies for biomedical research.
Ren’s team will map histone modifications and gene expression together at single cell resolution. Histone modifications are chemical changes to histone proteins, which make up cellular nucleosomes.
“By examining the histone modification together with gene expression,” said Ren, “we will be able to chronicle the state of genes and their regulatory elements for tens of millions of cells across the brain and over a lifespan.”
Ren’s team will also map the spatial distribution of hundreds of millions of brain cells.
“This information will define the structures and cellular composition of different neural circuits at unprecedented detail,” said Ren, “providing a reference for studying of a wide spectrum of neurological diseases.”
Earlier this year, Ren’s team was awarded a $10.6 million grant, in collaboration with colleagues at Sanford Burnham Prebys, to create a comprehensive atlas of how and where aging cells accumulate. The NIH grant is part of the Cellular Senescence Network program.
Currently, such detailed information, particularly regarding the human brain, is scant, almost non-existent.
“Essentially, we want to take millions, even hundreds of millions of brain cells, learn everything we can about their epigenetics and how their chromatin is arranged, and project them in a spatial context so we can see where these cells live, and understand how all of the cells in any brain region are organized, and at any age,” said Ecker.
Technology and data
When researchers typically study a single cell, the cell is removed from the rest of the brain, which limits the conclusions that can be drawn. To overcome this, researchers will employ a newer technique called spatial transcriptomics to distinguish cells by their genetic data and use that information to map the cells back to their original locations.
Specifically, the team will leverage a technology known as multiplexed error robust RNA FISH (MERFISH) that can detect and quantify RNA transcripts of hundreds of genes in each cell while also recording the anatomical location of the cell.
Ren’s laboratory recently invented a tool, known as Paired-Tag, that simultaneously maps histone modifications and gene expression from the same cells at very high throughput. The combined maps will allow the generation of integrative maps of histone modifications, gene expression, DNA methylation and chromatin architecture, for the cell types across the human brain and through the lifespan.
Ecker estimates that his lab alone will generate 11 petabytes of raw data during the project — the equivalent of 171,875 thumb drives (64GB each) — underscoring the center’s expertise and resources in computational biology, data storage and analysis. Washington University team members will be responsible for data management and integration.
“With the announcement of the BICAN awards, we are making an exciting transition in the overall BRAIN Initiative cell census program, which began in 2014,” said John Ngai, director of the NIH BRAIN Initiative. “These awards will enable researchers to explore the multifaceted characteristics of the more than 200 billion neurons and non-neuronal cells in the human brain at unprecedented detail and scale — a feat in advanced technologies and cross-team research collaboration that will reveal new paradigms for understanding how pathological changes in particular groups of brain cells could cause neurologic and neuropsychiatric disorders.”
About the NIH BRAIN Initiative
The NIH Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Initiative is a large-scale effort that seeks to deepen understanding of the inner workings of the human mind and to improve how we treat, prevent and cure disorders of the brain. Since its initial funding in 2014, the BRAIN Initiative has awarded more than $2.4 billion in research awards. The BRAIN Cell Census Network (mouse brain map) and BRAIN Cell Atlas Network (human brain map) are subsets of the BRAIN Initiative.
Read more:
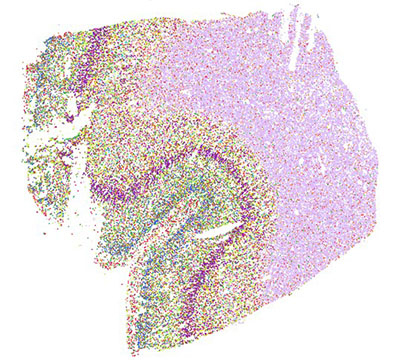
A cross-section of the middle temporal gyrus region of a male human brain that uses spatial transcriptomics, a technique that distinguishes cells by their genetic data and use that information to map the cells back to their original locations.