Julia Busiek, UC Newsroom

Cancer takes a staggering toll on American lives and families: Nearly 40 percent of people in the U.S. will be diagnosed with the disease at some point in their lives, and almost 1 in 5 Americans will die of it.
Thankfully, every day, scientists learn more about what causes cancer and how to prevent it, and they’re constantly finding and refining treatments and cures. Over decades, these insights have added up to hard-won progress: The overall cancer death rate in the U.S. has fallen by over 33 percent since 1991, which translates to roughly 3.8 million lives saved. And the pace of improvement continues to accelerate.
The American public has consistently supported this life-saving cancer research. The National Institutes of Health (NIH), the main federal agency for medical research, provides about $8 billion in cancer science funding annually in the U.S. This investment of taxpayer dollars has generated enormous return to the American people in the form of millions of lives saved and tens of millions more years lived, not to mention $69 billion in annual economic activity and 7 million jobs.
Now the nation’s bedrock source of public funding for biomedical research is under attack. Earlier this month the current administration announced significant cuts to NIH funding that academic and industry researchers alike rely on for essentials like lab space, equipment, safety and ethics.
“These cuts are a major hurdle to moving cancer research forward,” says Selma Masri, associate professor of biological chemistry at the UC Irvine School of Medicine. Masri’s research is tackling colorectal cancer, a disease that’s on the rise among young people, for reasons that scientists and clinicians don’t yet understand. In a pair of NIH-funded studies, Masri found that interrupting the body’s internal circadian clock caused changes to both the gut microbiome and the tumor immune response — suggesting potential links between sleep habits and biological rhythms with the rising rates of colorectal cancer in young adults.

These findings could inform advice to patients for preventing the disease or point to treatment strategies. But Masri says NIH funding cuts would halt progress before the promise of her research is realized. “NIH funding is essential to drive research productivity by supporting graduate students and postdoctoral fellows in my lab, in addition to covering administrative costs and maintaining research facilities on campus,” Masri says.
Learn more about links between circadian clock disruptions and cancer from UC Irvine
As the nation’s leader in NIH-funded research, the University of California is supporting legal action to block these cuts and avert dire consequences for patients and their families. UC President Michael V. Drake, M.D., called the cuts “catastrophic for countless Americans who depend on UC’s scientific advances to save lives and improve health care.”
Meanwhile, researchers at every UC campus and its five comprehensive cancer centers are continuing to push the field in bold new directions and extend life-saving treatments and prevention strategies to more people who need them. Read on to learn about some of the most recent NIH-funded cancer breakthroughs that have come out of UC labs.
Immune therapy emerges as the fourth pillar of cancer care

James Allison, who for 20 years was a UC Berkeley immunologist conducting fundamental research on cancer, is now at the M.D. Anderson Cancer Center in Houston, Texas.
Much recent cancer research has sought to expand the field of immune therapy, which aims to boost our body’s natural defenses to attack cancer, instead of poisoning cancer with chemotherapy. Cancer immune therapy originated in part at UC Berkeley, where a scientist named James Allison used funding from NIH to conduct basic research on how the immune system — in particular, the T cell — fights infection.
These discoveries laid the foundation for cancer immune therapy, which “now has taken its place along with surgery, chemotherapy and radiation as a reliable and objective way to treat cancer,” Allison said in 2015. Allison, who presently works at the University of Texas MD Anderson Cancer Center in Houston, earned the 2018 Nobel Prize in medicine for his role in developing the so-called fourth pillar of cancer care.
More about Dr. Allison's research from UC Berkeley
A tweak to “natural killer” immune cells helps them fight cancer
Another immune-based strategy, one that uses engineered T cells from cancer patients, “has been very good in treating certain blood cell cancers, certain types of leukemia, lymphoma, and myeloma,” says Dan Kaufman, professor at the UC San Diego School of Medicine and director of cell therapy at the UC San Diego Moores Cancer Center. “Where the field has been challenged, and where the next goal is, is treating solid tumors such as liver cancer, lung cancer, breast cancer and colon cancer, which have a much higher incidence than those blood cell cancers.”
Kaufman recently advanced the fight against a type of liver cancer called hepatocellular carcinoma. It’s the fifth-most common and the second-deadliest form of cancer, killing more than 4 in 5 patients within five years. The disease is so deadly in part because its tumors produce certain proteins that directly inhibit the function of our body’s immune cells, weakening our defenses and allowing the cancer to grow relatively unchecked. And experiments using modified T cells to treat the cancer have so far had “relatively modest results,” Kaufman says.
Kaufman’s team turned their focus to another part of the immune system known as natural killer (NK) cells. Previous work had identified the specific receptor on NK cells that binds with the harmful protein produced by tumors. So Kaufman’s team engineered a line of NK cells without that receptor. His group had created a way to produce these engineered NK cells relatively quickly and affordably in a lab using stem cells. They then injected these engineered cells into a tumor site. With nowhere to bind, the would-be immunosuppressive proteins were no longer able to block the function of the modified NK cells, leaving the NK cell intact to attack the tumor. The treatment produced “prolonged survival in a mouse model using human tumor cells and the engineered human NK cells,” Kaufman said.
More about advances in NK-cell therapy from UC San Diego
In a ‘judo move,’ using cancer’s strength against it
Meanwhile, a team at UC San Francisco has discovered a strategy to strengthen T cells in their fight against solid tumors. They zeroed in on what happens when the body’s naturally occurring T cells turn malignant, going from defending against disease to causing cancer.
In an NIH-funded study, the team identified the particular mutation that makes malignant T cells especially adept at spreading lymphoma through the body. The team took the gene for this mutation from malignant cells and inserted it into healthy cells — a simple step that boosted these normal cells’ power to do their cancer-killing job by 100x. These modified T cells kept tumors at bay for many months, with little evident harm to healthy cells or tissues.

Kole Roybal, associate professor at UC San Francisco and the UCSF Helen Diller Family Comprehensive Cancer Center.
The experiment was inspired by the martial arts principle of using an opponent’s strength against them, said coauthor Kole Roybal, associate professor at UC San Francisco and the UCSF Helen Diller Family Comprehensive Cancer Center. “We’ve used the mutations that give cancer cells their staying power to engineer what we call a ‘Judo T-cell therapy’ that can survive and thrive in the harsh conditions that tumors create,” said Roybal. The team has already begun working toward testing this new approach in people.
Another team at UC San Francisco recently received funding from the NIH for the second phase of a clinical trial that will test the use of new immunotherapy techniques for patients with glioblastoma, the most common and deadly adult brain tumor.
Finding the off switch to stunt pancreatic cancer’s rapid growth
In 2022, chemists at UC Berkeley studied another type of immunotherapy against pancreatic cancer, one of the deadliest of cancers, with a five-year survival rate of just 10 percent. A team of scientists at the Chang Lab found that the spread of pancreatic cancer throughout a patient’s body is triggered by the loss of an enzyme that repairs oxygen damage. Absent this reparative enzyme, pancreatic cancer cells rev up and rapidly seed new cancers. Now, researchers are looking at other types of cancer for evidence of the trigger they discovered, and they’re devising ways to boost levels of the enzyme in hopes of preventing the metastatic spread of pancreatic cancer cells.
Taming the ‘chaotic’ protein that fuels 3 in 4 types of cancer
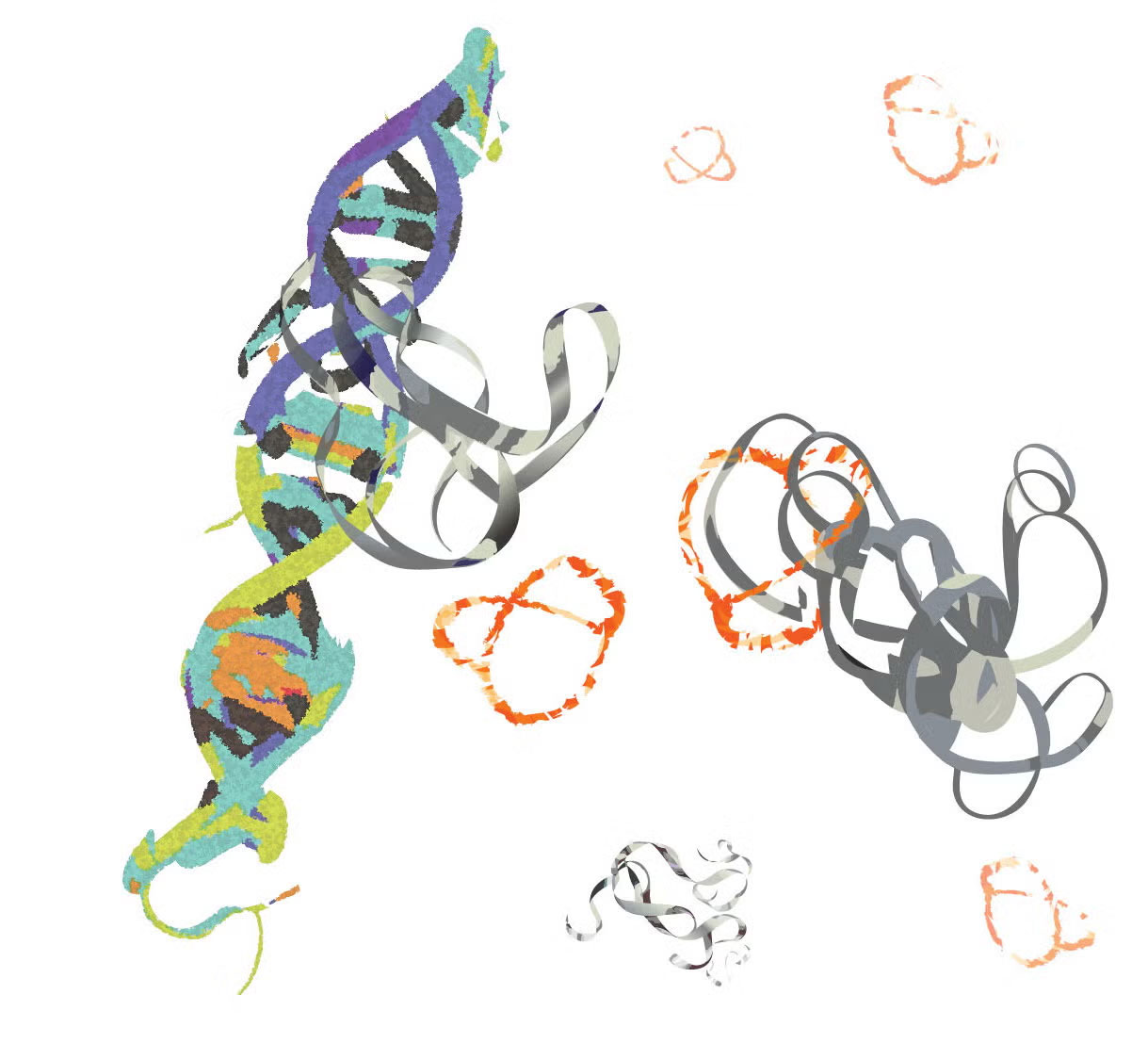
MYC proteins (grey ribbons) bind to DNA and promote cancer progression. Researchers at UC Riverside developed a molecule (orange pretzel-like shape) that binds to MYC, inhibiting its cancer-promoting function.
In healthy cells, a protein called MYC helps guide the process of transcription, in which genetic information is converted from DNA into RNA. But when cells turn cancerous, they start to produce way too much MYC, fueling the rapid multiplication of cancerous cells. This MYC overload is a factor in up to 75 percent of all human cancer cases, and the protein’s peculiarly chaotic structure has so far stymied medicine’s attempts to control it.
MYC is “basically a glob of randomness,” said UC Riverside associate professor of chemistry Min Xue. “Conventional drug discovery pipelines rely on well-defined structures, and this does not exist for MYC.”
Last year, Xue’s team published an NIH-funded study describing a win in the fight against MYC’s tricky structure. The discovery revolves around peptides, the molecular building blocks of proteins. The team found that altering the rigidity and shape of a certain peptide helps it bind to MYC and prevent it from fueling the growth of cancer cells. Ultimately, they hit on a novel two-peptide combo that was about a hundred times more effective at targeting and interrupting MYC. Now, they’re working to develop chemistry that improves the peptides’ ability to get inside cells, the next step toward developing a drug based on this research.
Protect life-saving research powered by NIH funding
These promising findings are just the latest results of a longstanding partnership between the federal government and UC scientists. Over generations, this partnership has yielded blockbuster advancements toward the first flu vaccine, the discovery of the role of LDL and HDL cholesterol in heart disease, the invention of modern gene editing, and much, much more.
The cuts to NIH funding proposed by the current administration “will threaten American lives, disrupt time-sensitive life-saving research, and cripple our innovative knowledge-based economy,” said Theresa Maldonado, UC vice president for Research and Innovation.
“Cancer is an awful disease, and we need to continue pushing to find preventions and cures for it,” says professor Masri at UC Irvine. “The only way to move that forward is to learn about the disease, to learn about the mechanistic underpinnings, and then to come up with modalities to target it.”

