Scott LaFee, UC San Diego

Researchers at University of California San Diego School of Medicine say the loss of a single gene two to three million years ago in our ancestors may have resulted in a heightened risk of cardiovascular disease in all humans as a species, while also setting up a further risk for red meat-eating humans. The findings are published July 22, 2019 in PNAS.
Atherosclerosis — the clogging of arteries with fatty deposits — is the cause of one-third of deaths worldwide due to cardiovascular disease. There are many known risk factors, including blood cholesterol, physical inactivity, age, hypertension, obesity and smoking, but in roughly 15 percent of first-time cardiovascular disease events (CVD) due to atherosclerosis, none of these factors apply.
A decade ago, Nissi Varki, M.D., professor of pathology at UC San Diego School of Medicine, with co-author Ajit Varki, M.D., Distinguished Professor Of Medicine and Cellular And Molecular Medicine, and colleagues noted that naturally occurring coronary heart attacks due to atherosclerosis are virtually non-existent in other mammals, including closely related chimpanzees in captivity which share human-like risk factors, such as high blood lipids, hypertension and physical inactivity. Instead, chimp “heart attacks” were due to an as-yet unexplained scarring of the heart muscle.
In the new study, the Varkis, and Philip Gordts, Ph.D., assistant professor of medicine, and others report that mice modified to be deficient (like humans) in a sialic acid sugar molecule called Neu5Gc showed a significant increase in atherogenesis compared to control mice, who retain the CMAH gene that produces Neu5Gc.
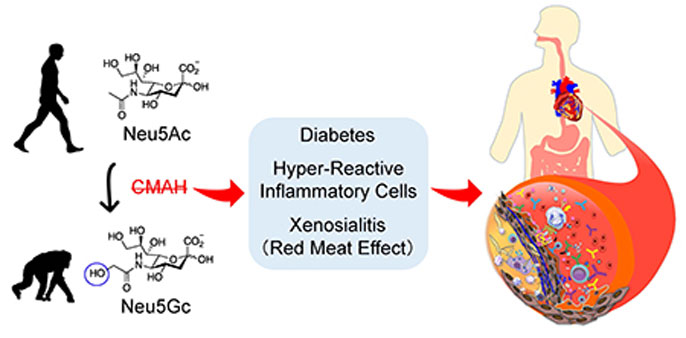
The researchers — members of the Glycobiology Research and Training Center and/or the Center for Academic Research and Training in Anthropogeny at UC San Diego — believe a mutation that inactivated the CMAH gene occurred a few million years ago in hominin ancestors, an event possibly linked to a malarial parasite that recognized Neu5Gc.
In their findings, the research team said human-like elimination of CMAH and Neu5Gc in mice caused an almost 2-fold increase in severity of atherosclerosis compared to unmodified mice.
“The increased risk appears to be driven by multiple factors, including hyperactive white cells and a tendency to diabetes in the human-like mice,” said Ajit Varki. “This may help explain why even vegetarian humans without any other obvious cardiovascular risk factors are still very prone to heart attacks and strokes, while other evolutionary relatives are not.”
But in consuming red meat, humans are also repeatedly exposed to Neu5Gc, which researchers said prompts an immune response and chronic inflammation they call “xenosialitis.” In their tests, human-like mice modified to lack the CMAH gene were fed a Neu5Gc-rich, high-fat diet and subsequently suffered a further 2.4-fold increase in atherosclerosis, which could not be explained by changes in blood fats or sugars.
“The human evolutionary loss of CMAH likely contributes to a predisposition to atherosclerosis by both intrinsic and extrinsic (dietary) factors,” wrote the authors, “and future studies could consider using this more human-like model.”
Red meat and cancer
Courtesy of Kunio Kawanishi
In previous work, the Varkis and colleagues have shown that dietary Neu5Gc also promotes inflammation and cancer progression in Neu5Gc-deficient mice, suggesting that the non-human sugar molecule, which is abundant in red meat, may at least partially explain the link between high consumption of red meat and certain cancers.
The loss of NeuG5c in humans (retained in other primates) increases atherosclerosis risk by multiple mechanisms, including intrinsic factors such as heightened inflammatory response and hyperglycemia and extrinsic factors such as red meat-derived Neu5Gc-induced xenosialitis. Graphic courtesy of Kunio Kawanishi.
Interestingly, the evolutionary loss of the CMAH gene appears to have produced other significant changes in human physiology, including reduced human fertility and enhanced ability to run long distances.
Co-authors include: Chirag Dhar and Raymond Do, UC San Diego and first author Kunio Kawanishi, UC San Diego, now at University of Tsukuba, Japan.
Funding for this research came, in part, from the National Institutes of Health (grant R01GM32373), the American Heart Association Postdoctoral Fellowship (17POST33671176) and the Foundation Leducq (16CVD01).

