Alex Russell, UC Davis

Women are nearly twice as likely as men to be diagnosed with an anxiety disorder, but among boys and girls the likelihood is the same. New University of California, Davis, research has identified changes in the brain during puberty that may account for differences in how women and men respond to stress.
A team of psychologists has found that testosterone is the key hormone that drives gender-based differences in responses to social stress. The study encompassed six separate experiments with mice to isolate what changes in the brain drive these differences between males and females. The study was published in the Proceedings of the National Academy of Sciences, or PNAS, today.
“This research shows how the body’s hormones shape the complex interplay between the brain’s circuitry and behavioral responses to stress,” said Brian Trainor, a professor of psychology in the College of Letters and Science at UC Davis and the study’s corresponding author.
How male and female mice respond to stress
To better understand how changes in the brain during puberty affect an individual’s stress response, the research team placed mice into a series of encounters with another, more aggressive mouse.
Over time, adult female mice were far more likely than adult males to avoid new, different mice. Adult males acted the same with an unfamiliar mouse whether or not they had experienced the stress of encountering a more aggressive mouse.
However, when the team conducted the same experiment with younger male and female mice, there were no differences in how they reacted to unfamiliar mice. Both males and females were roughly equally as wary if they had encountered a more aggressive mouse.
“Between puberty and adulthood, something changes,” said Emily Wright, a postdoctoral fellow at UC Davis who led the study at Trainor’s lab. “Before puberty, both males and females become more timid and unwilling to interact with new mice after the stress exposure.”

A breakthrough with implanted testosterone
To test the idea that the change itself took place during puberty, the team removed the testes of male mice before puberty began. These mice then grew up without exposure to male hormones — including testosterone. As adults, these mice showed the same responses to stress in the experiment as female adult mice.
One of the study’s breakthroughs came when the team used an implant that replaced only dihydrotestosterone, a strong version of testosterone, in males that had their testes removed before puberty. The team also gave dihydrotestosterone implants to females during puberty to see if the hormone would also alter their stress response.
On its own, the dihydrotestosterone implants caused no immediate behavioral changes in males or females. However, after exposure to the social stress of an aggressive mouse, the effect was clear: Mice that had received the dihydrotestosterone implants showed almost no effect of stress.
With the implants, both males and females behaved just like adult males that had gone through puberty.
“Dihydrotestosterone by itself does nothing,” said Wright. “But once placed in a stressful situation the effect became clear.”
A look into the brain under stress
Having shown that testosterone was the key difference between males and females in terms of their responses to social stress, the team then set out to test how that hormone changed brain function during puberty using cutting-edge neuroimaging technology.
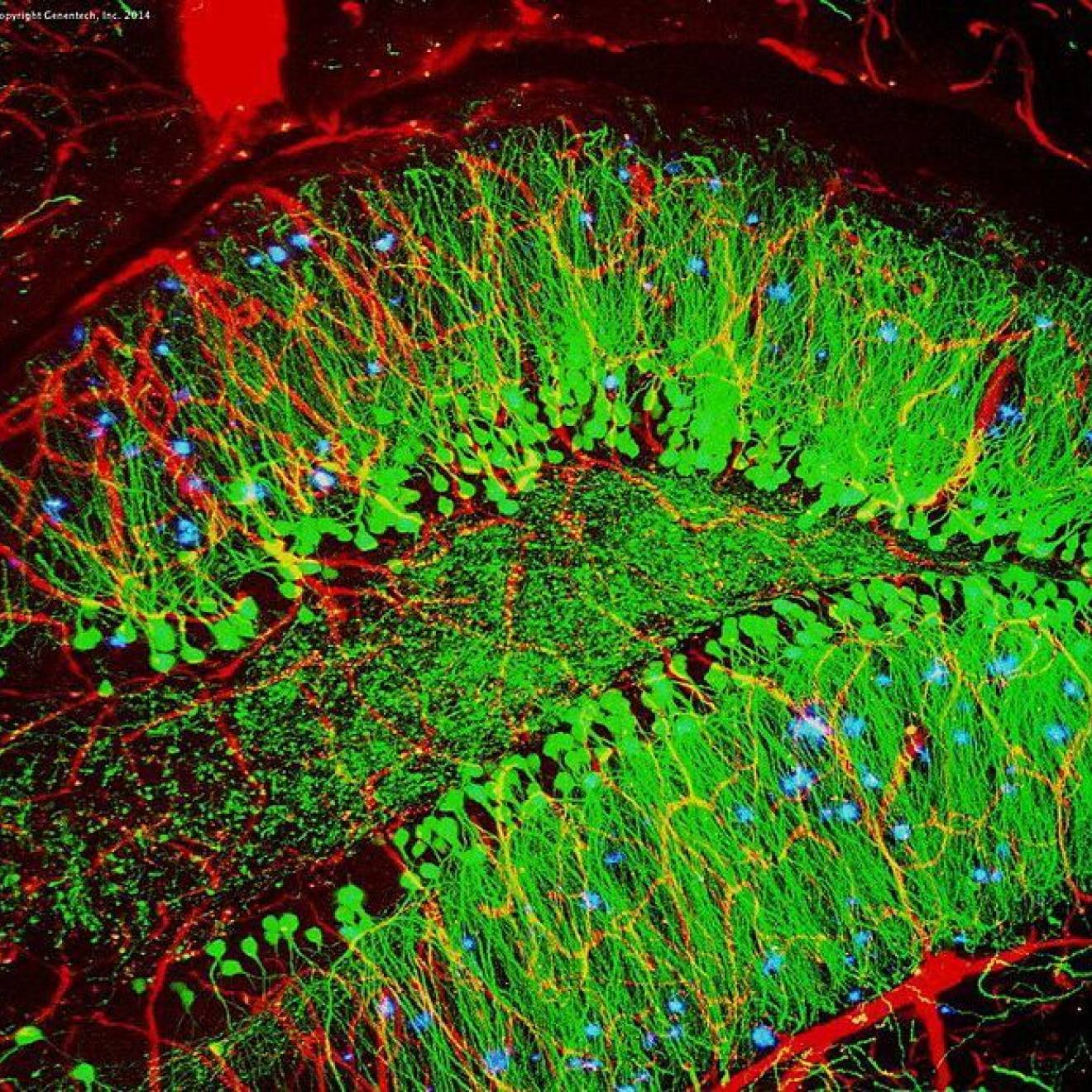
The team implanted a fiber-optic wire into the amygdala, which is the part of the brain most responsible for stress responses. The implant included a calcium-triggered biosensor that produces green light when neurons are active. Increases and decreases in green light indicated neuron activity in real time.
The implant revealed unusually high levels of brain activity in the amygdala when mice that had never been exposed to testosterone — male or female — were in close proximity to unknown mice. However, mice that had been exposed to testosterone showed no increase in that neural activity.
“When a mouse is interacting with a potential aggressor, we saw an increase in activity in the brain’s extended amygdala, which suggests a threat signal,” said Wright. “This response is exaggerated in mice that grow up without testosterone.”
An understanding of stress
Though the experiments were with mice, the neural pathways and hormones the team identified could help interpret data from studies in humans, Trainor said. Humans can also shy away from others and watch them from afar, he said.
“For more than a decade we’ve known that female mice respond to stress by avoiding new situations,” said Trainor. “This study shows the biological mechanisms behind those responses.”
Trainor said there’s a lot of urgency to study how mental health differs between men and women. As recently as 15 years ago, he said, even animal models of anxiety and depression primarily focused on males.
“The effect of stress is not the same in males and females,” said Wright. “That’s why we need to study it.”
The paper was co-authored by researchers from the UC Davis School of Medicine, the Wisconsin National Primate Research Center and the California National Primate Research Center.
The research was supported by the National Institutes of Health and the National Science Foundation.